gcsescience.com 17 gcsescience.com
Elements, Compounds and Mixtures
Why is Diffusion in a Liquid Slow?
Potassium Manganate
(VII) is dark
purple.
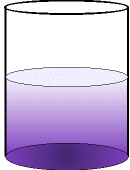
When the solid is dissolved in water,
the purple particles slowly diffuse out into the clear
liquid.



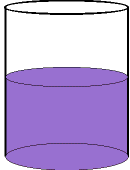
Eventually, the random motion of all
the particles
results in the purple colour being
equally
dispersed throughout the water.
Diffusion appears to be slow because the dissolved
particles
of the potassium manganate
(VII) have many collisions
with
water
molecules and each
other. This slows their progress.
![]() Links
Solid
Liquid
Gas
Revision Questions
Links
Solid
Liquid
Gas
Revision Questions
![]()
gcsescience.com The Periodic Table Index Chemistry Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.