gcsescience.com 39 gcsescience.com
What are the Isomers of Pentanol?
There are different isomers of the alcohols
propanol, butanol and pentanol.
The eight isomers of pentanol are shown below.
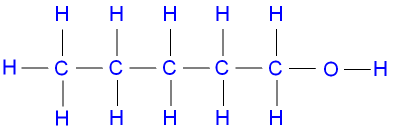
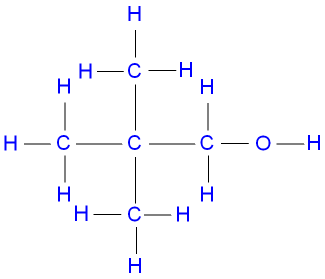
Pentanol
(C5H11OH) is commonly represented
by the molecule pentan-1-ol,
a primary
alcohol, which has the structural formula

Other isomers of pentanol
can be drawn by changing
the way that
the
O–H
group is joined to the
molecule
or by changing the way that the carbon atoms
are joined to each other (see the isomers of pentane).
The other isomers of pentanol
are pentan-2-ol,
a secondary alcohol,
represented by the structural formula
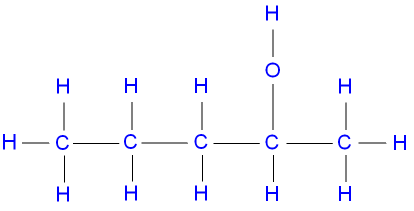
pentan-3-ol, a secondary alcohol,
represented below by the structural formula
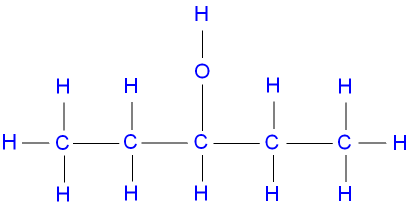
2-methylbutan-1-ol, a primary alcohol,
represented below by the structural formula
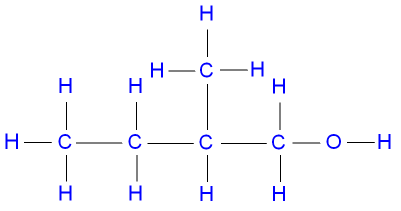
3-methylbutan-1-ol, a primary alcohol,
represented below by the structural formula
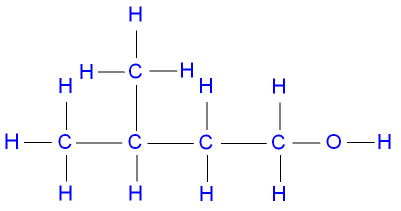
2-methylbutan-2-ol, a tertiary alcohol,
represented below by the structural formula
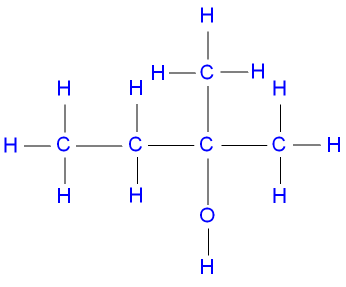
2-methylbutan-3-ol (or 3-methylbutan-2-ol)
a secondary alcohol,
represented below by the structural formula
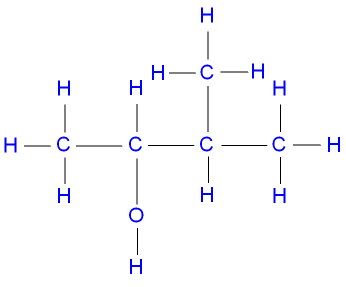
2,2-dimethylpropanol, a primary alcohol,
represented below by the structural formula
The single lines between the atoms
represent single covalent bonds.
Note that each carbon atom has
four bonds
(valency 4),
each oxygen atom has two bonds
(valency 2)
and each hydrogen atom has one bond
(valency 1).
Valency is the combining power of an atom.
![]() Links Alcohols Revision Questions
Links Alcohols Revision Questions ![]()
gcsescience.com The Periodic Table Index Alcohol Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.