gcsescience.com 20 gcsescience.com
Radiodating.
What is Radiodating?
The half-life of a
radioisotope can be used to measure
the
age of things.
The method is called radiodating.
Radiodating
can be used to measure the age
of rocks (see below)
and carbon dating
can be used to date archaeological specimens.
Using Uranium-238 to Date
Rock.
Some rocks contain uranium-238 which is radioactive and
follows a decay series until it
produces a stable isotope of lead.
The amount of uranium in the rock
is compared to the
amount of
lead and then the age of the rock can be calculated.
For example, it is found
that there are equal
amounts of uranium and
lead in a rock.
The half-life
of uranium-238 is
4·5 billion years.
After 4·5
billion years, half of the
uranium originally present
in the rock would have decayed and become lead.
The proportion of uranium to lead would
be 1 to 1
(equal amounts).
The rock could therefore be
dated as 4·5 billion years
old.
You can only use the ratio of uranium-238 to lead
to date rock if
you
are sure that there was no lead originally present in the rock and
that all the lead in the rock has come from the decay of uranium.
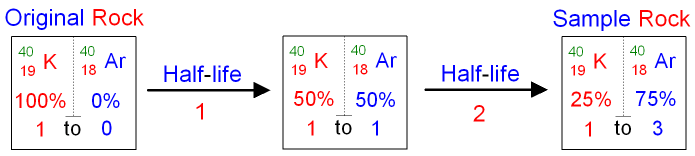
Using Potassium-40 to Date
Rock.
Some rocks contain the radioisotope
potassium-40 which
decays to form
argon-40. Argon-40 is a stable isotope.
If the argon gas is unable to escape from
the rock, then the
proportions of potassium-40 to
argon-40 can be
used to date the rock.
For example, it is found that
there is three times
as much argon-40 as
potassium-40 in a
rock.

After 2
half-lives
there is three
times as much argon-40
as potassium-40 in the rock (see the boxes above).
The half-life of potassium-40 is
1·3 billion years.
The rock could therefore be
dated as 2 x 1·3
billion years
= 2·6
billion years
old.
![]() Links
Radioactivity
Half-life
Revision Questions
Links
Radioactivity
Half-life
Revision Questions
![]()
gcsescience.com Physics Quiz Index Radioactivity Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.