2H2O2(aq)
gcsescience.com 14 gcsescience.com
What is the Effect of using a Catalyst
for the Decomposition
of Hydrogen Peroxide?
At room
temperature hydrogen
peroxide decomposes
very slowly.
The presence of a catalyst may cause it to decompose
quickly.
hydrogen
peroxide ![]() oxygen + water.
oxygen + water.
2H2O2(aq)
![]() O2(g) + 2H2O(l)
O2(g) + 2H2O(l)
The rate of this
reaction
can be followed by
recording the volume of
oxygen as it is produced
and plotting a graph
of volume against time
as
shown below. You must know a test for oxygen gas.
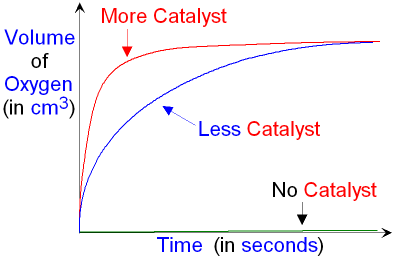
What is the Effect of using Different
Amounts of Catalyst
for the Decomposition
of Hydrogen Peroxide?
The catalyst used is manganese(IV)
oxide - MnO2(s)
Using more catalyst will show an
increase in reaction rate.
This is because more catalyst will
have a
greater surface area for
the reaction to take place.
What is the Effect of using Different
Catalysts
for the Decomposition
of Hydrogen Peroxide?
The reaction can be performed using different
catalysts to
compare how
well each catalyst works for
the same reaction.
A fair test must use the same amount of each catalyst.
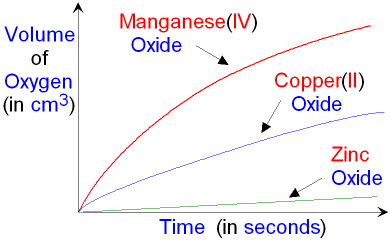
As you can see from the
graph, manganese(IV) oxide
(MnO2(s))
is
the best catalyst for
this reaction. The gradient of the
plot
for manganese(IV) oxide
is greater
(steeper) than the other two.
Copper(II) oxide
(CuO(s)) is a less effective catalyst
than manganese(IV) oxide. Zinc oxide (ZnO(s)) is
the least effective of the three catalysts for this
reaction.
![]() Links
Catalysts and Energy
Enzymes
Revision Questions
Links
Catalysts and Energy
Enzymes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Catalyst Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.