C2H5OH(aq) + O2(g)
gcsescience.com 49 gcsescience.com
Ethanoic Acid.
What is Ethanoic Acid?
When ethanol
reacts with oxygen it forms a weak acid
called
ethanoic acid. In an open bottle of beer or wine, the
reaction happens naturally in the
presence of bacteria, and it
is the ethanoic acid that can make beer or wine taste sour.
ethanol +
oxygen ![]() ethanoic acid + water.
ethanoic acid + water.
C2H5OH(aq)
+ O2(g) ![]() CH3CO2H(aq) + H2O(l)
CH3CO2H(aq) + H2O(l)
Ethanoic
acid is a weak acid.
It is found in vinegar ("vinegar" is old french for "sour wine").
Vinegar is used as a food flavouring and preservative.
Ethanoic
acid is used to make a polymer called acetate
rayon.
Acetate rayon fibres can be woven to make clothing and fabrics.
What is the Structure of Ethanoic
Acid?
The structure of ethanoic acid is shown in the picture below.
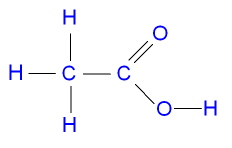
Ethanoic
acid will
react
with alcohols, alkalis, carbonates and metals.
![]() Links Ethanoic Acid Revision Questions
Links Ethanoic Acid Revision Questions
![]()
gcsescience.com The Periodic Table Index Carboxylic Acid Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.