gcsescience.com 77 gcsescience.com
Vegetable Oils - Emulsifiers.
What is an Emulsifier?
An emulsifier can
make an emulsion more
stable
by
making the oil droplets
stay in the water for
a longer time.
Examples of food emulsifiers
are mustard and
egg yolk (the yellow bit
in the middle).
Mayonnaise is an emulsion
of oil and water
using
egg yolk as an emulsifier.
Mayonnaise also contains lemon
juice or vinegar.
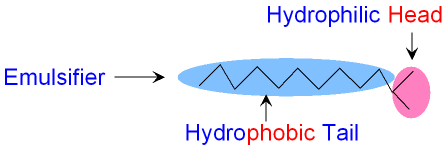
How does an Emulsifier work?
An emulsifier molecule
works by having two parts.
One part of the molecule
has an electric charge
(like
an ion)
and will dissolve in water but not in oil.
This part of the
molecule (known as the head) is
called hydrophilic
which means water loving.
The other part (called the tail) is a long
hydrocarbon
which will dissolve in oil
but not in water. This
part of the
molecule is called hydrophobic
which means
water fearing. The picture
below shows the hydrophilic
head and the hydrophobic
tail of an emulsifier
molecule.

The emulsifier molecule dissolves with its head
in
the
water
and its tail in the oil
droplet.
A large number
of
emulsifier molecules are needed to keep the
oil
droplet
dispersed in the water
for a long time.
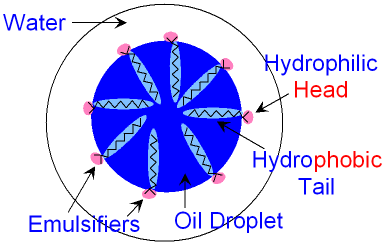
The picture above shows how the emulsifier molecules
position
themselves to make an oil droplet more
stable.
gcsescience.com The Periodic Table Index Oil Products Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.