gcsescience.com 82 gcsescience.com
Fuels - Hydrogen Fuel Cell.
What is a Fuel Cell?
A fuel cell is not
the same as a battery.
A fuel cell
needs to be continuously supplied with both
a fuel
and oxygen, which react together
and produce electricity.
When the fuel
being used is hydrogen
the fuel cell
produces electricity
plus water.
What is a Hydrogen Fuel Cell?
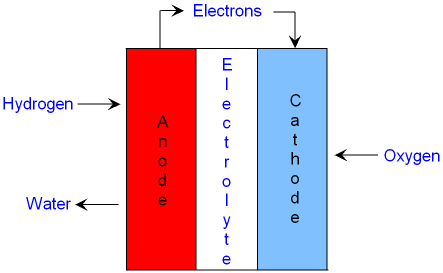
A hydrogen
fuel cell is made from an anode and a cathode
with an electrolyte contained
between them.
The fuel cell generates
electricity. It is not
the same as an
electrolysis cell which needs to be supplied with electricity.

The electrolyte in an alkali fuel cell is potassium hydroxide (KOH).
The anode is a platinum
catalyst.
Hydrogen is supplied to
the anode and it reacts with hydroxide
ions from the
electrolyte to make water.
This is an oxidation
reaction
in which electrons are lost from hydrogen.
The electrons
leave the anode and travel through the external circuit
which is using
the electricity that is supplied
by the fuel cell.
The cathode is
also a platinum catalyst.
Oxygen supplied to
the cathode
reacts with water and
gains electrons to make
hydroxide
ions in the electrolyte. This is a reduction
reaction.
The overall reaction in the fuel cell is
hydrogen + oxygen
![]() water + energy
water + energy
2H2(g)
+ O2(g) ![]() 2H2O(l)
2H2O(l)
The electricity provided
by the fuel cell can be used
to run an electric vehicle
or to power other equipment.
What are the Advantages of a Hydrogen
Fuel Cell?
1. It can be a renewable source of electricity
if
the hydrogen
comes from a renewable resource.
See the advantages
of hydrogen as a fuel.
2. It does not
produce pollution or contribute
to global warming
because the only product is water.
What are the Disadvantages of a Hydrogen
Fuel Cell?
1. Each fuel cell
only makes a small voltage.
A large number of fuel cells must be
wired together to
produce a large voltage
or current. This is expensive.
2. Hydrogen is explosive and difficult to store.
3. If the hydrogen
does not come from a renewable
resource
but is made from methane, then
the electricity
produced from the fuel cell
is also not renewable.
![]() Links Fuels Revision Questions
Links Fuels Revision Questions ![]()
gcsescience.com The Periodic Table Index Oil Products Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.