gcsescience.com
3
gcsescience.com
Energy Transfer
What is the Difference
between Heat
and Temperature?
What is Heat?
Heat
is a form of energy and is measured
in joules.
Scientists use the word "thermal" to mean "heat".
Heat
energy and thermal energy both mean the same thing.
What is Temperature?
Temperature is a measure of how hot something
is
and is measured in °C (degree
Celsius).
Heat is related to temperature but the two are not the
same.
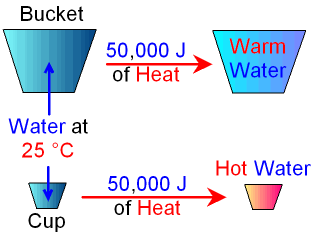
Imagine that you have a
bucket full of water and a cup full of water
both at 25 °C.
If you add the same amount of heat
energy
(for example 50,000J) to both you would find that
the temperature of
the cup of water
increases by
much more than the temperature of the bucket of water.
Temperature is a
measure of the kinetic energy of
the particles
(the faster they are going, the hotter they are).
Temperature does not depend on the mass of the substance
(how many particles there are).
The amount of heat energy that a
substance has
does depend on its mass.
If you double the mass, you must double
the heat energy
to heat it to the same temperature.
The amount of heat energy that a
substance has
also depends on its specific heat capacity.
What is Specific Heat
Capacity?
The specific heat capacity is the amount
of heat energy
needed to raise 1 kg of a substance
by 1
°C.
Heat transferred =
specific heat capacity x mass x change in temperature.
E = c x
m x θ
Where E
is the energy
(heat) transferred in J
c is the specific heat capacity
in J / kg °C
m is the mass in kg
θ
(pronounced "theeter") is the change in temperature in °C .
This means that the same mass
of different
substances
will contain different amounts
of heat energy
even though they are at the same temperature.
 Links
Energy
Transfer
Revision Questions
Links
Energy
Transfer
Revision Questions
gcsescience.com
Physics Quiz
Index
Heat Quiz
gcsescience.com
Home
GCSE Chemistry
GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.
