gcsescience.com 22 gcsescience.com
Carbon Dating and Archaeological Specimens.
What are Archaeological Specimens?
Archaeological specimens are things which belonged to
people in the past and have been dug up from the
ground.
Archaeological is pronounced
ar-key-o-logical.
Why are Archaeological Specimens Important?
The study
of archaeological specimens can tell you
a lot
about how
people in the past lived. One of the most important
things
to
know is the age of the object. This tells you how
long ago the
thing was used or how long ago the
thing was living.
How is Carbon
Dating used to Date Archaeological Specimens?
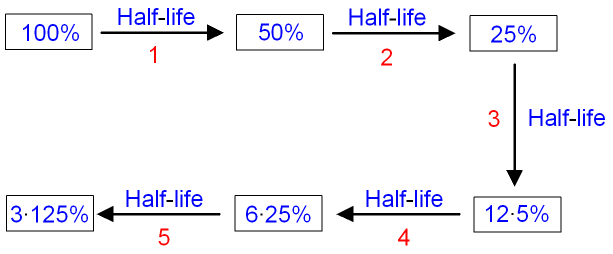
The half-life of carbon-14 is
5,730 years. The half-life
can be
used to calculate the age of a sample
containing carbon-14.
Carbon dating
is very useful but also has
its limitations.
Question.
If a specimen sample had an amount of carbon-14 which
was
25%
of the amount in today's environment, how old would the sample
be?
Answer.
Find how many half-lives
would be needed to reduce the amount of carbon-14 to
25%.

After 2 half-lives the amount of carbon-14 is reduced to 25%.
The sample is 2 x 5,730 years
old
=
11,460 years old.
If the amount of carbon-14 in the sample
was only
6·25%,
then the sample would be 4 half-lives
old.
4 half-lives are 4 x 5,730 years
= 22,920
years old.
![]() Links
Radioactivity
Half-life
Revision Questions
Links
Radioactivity
Half-life
Revision Questions
![]()
gcsescience.com Physics Quiz Index Radioactivity Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.