gcsescience.com 26 gcsescience.com
How does a Smoke Detector Work?
How is Radioactivity used in a Smoke Detector?
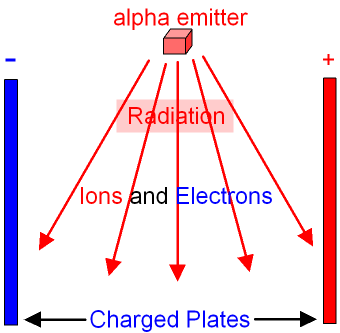
The radioactive source in a smoke detector is americium-241.
Americium-241 is an alpha emitter. The alpha
particles
ionise the molecules
of the air forming
ions and electrons
which are attracted to a pair
of charged plates as shown in the
picture below. When the ions and
electrons hit the
plates
they
provide a small amount of
electricity
(a small current).
This
current stays constant
and is monitored by the
smoke alarm.

What happens when Smoke Enters the Smoke Detector?
When smoke enters the smoke detector, the smoke
particles
near to the radioactive source
absorb many of the
alpha particles
before they can ionise the air
between the charged plates.
The number of ions and electrons
between the charged plates
therefore decreases and so the current
which is passed
between the charged
plates
also decreases. The
smoke
detector notices the
decrease in current and sets off
the alarm.
![]() Links
Radioactivity
Revision Questions
Links
Radioactivity
Revision Questions
![]()
gcsescience.com Physics Quiz Index Radioactivity Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.