gcsescience.com 11 gcsescience.com
The Alkali Metals - Electrolysis of Sodium Chloride in Water.
This page shows the electrolysis of sodium chloride
which has been dissolved in water.
You get
different products if the
sodium chloride is completely dry.
What is the Electrolysis of Brine?
Sodium chloride
dissolved in water is called
brine.
Electrolysis of
brine gives hydrogen gas at the cathode and
chlorine gas at the anode.
You must know how to test
for hydrogen and chlorine gas.
Sodium hydroxide remains
dissolved in the solution.
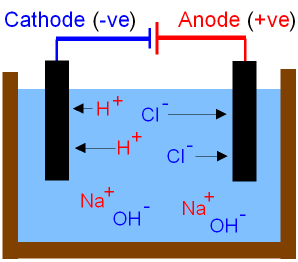
The picture below shows
what happens to
the ions during the electrolysis
of brine.
This is an important industrial
process making
hydrogen gas, chlorine
gas and sodium
hydroxide.

What are Half Equations?
The reactions at each electrode are called half
equations.
The half equations are written so that
the same number of electrons
occur in each equation.
2H+ + 2e- ![]() H2 (hydrogen gas at the (-)cathode).
H2 (hydrogen gas at the (-)cathode).
2Cl- -
2e- ![]() Cl2 (chlorine gas at the (+)anode).
Cl2 (chlorine gas at the (+)anode).
Hydrogen
ions gain
electrons (reduction) to form
hydrogen atoms.
The hydrogen atoms combine to form molecules
of hydrogen gas.
Chloride ions lose
electrons (oxidation) to form
chlorine atoms.
The chlorine atoms combine to form molecules
of chlorine gas.
The overall
reaction is
2NaCl(aq) + 2H2O(l) ![]() 2Na+(aq) + 2OH-(aq) + Cl2(g) + H2(g)
2Na+(aq) + 2OH-(aq) + Cl2(g) + H2(g)
See more detail of the electrolysis on the next page.
The electrolysis of hydrochloric
acid also gives
hydrogen at the cathode and chlorine
at the anode but
hydrochloric acid is more
expensive than brine
and you do not get sodium
hydroxide left in the solution.
![]() Links
Alkali Metals
Revision Questions
Links
Alkali Metals
Revision Questions
![]()
gcsescience.com The Periodic Table Index Periodic Table Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.