2HCl(aq) + Na2S2O3(aq)
gcsescience.com 8 gcsescience.com
What is the Effect of Increasing the Concentration
on the Rate of a Chemical Reaction?
If the concentration of a substance is increased
then
there will be more
particles of the substance per
dm3 in
the solution.
The more particles that
there are in the
same volume, then the
closer to each other the particles
will be.
This means that the particles will collide
more frequently
and the rate of the
reaction increases.
The effect of increasing the
concentration on the rate of
a reaction can be shown by looking at the reaction between
sodium thiosulfate solution and dilute hydrochloric acid.
The balanced
equation for this reaction is shown below.
HCl + sodium thiosulfate ![]() sodium chloride + sulfur
dioxide + sulfur + water.
sodium chloride + sulfur
dioxide + sulfur + water.
2HCl(aq) + Na2S2O3(aq) ![]() 2NaCl(aq) + SO2(g)
+ S(s)
+ H2O(l)
2NaCl(aq) + SO2(g)
+ S(s)
+ H2O(l)
Solid
sulfur (S(s)) is formed in the
flask as one of the products.
Increasing the concentration of sodium thiosulfate means
that the solid sulfur will be produced more quickly and there
will be less time before the cross can
no longer be seen.
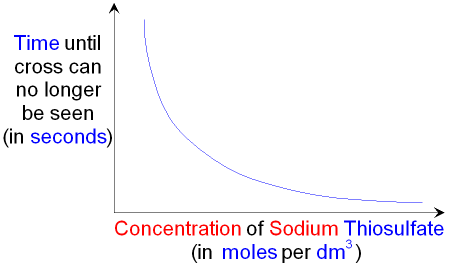
When making different concentrations of sodium thiosulfate
solution for this experiment, a fair test
must use the
same total volume
of sodium thiosulfate plus hydrochloric acid.
![]() Links
Catalysts and Energy
Enzymes
Revision Questions
Links
Catalysts and Energy
Enzymes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Reaction Rate Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.