2HCl(aq) + CaCO3(s)
gcsescience.com 10 gcsescience.com
What is the Effect of Increasing the Surface Area
of a Solid on the Rate of a Chemical Reaction?
A solid that is present in a solution
can only react
when particles collide with the surface of the solid.
If the area of the solid
surface is made bigger
then more particles can collide
with it per second
and the rate of the
chemical reaction increases.
You can increase the surface
area of a solid by breaking
it down into smaller pieces (see also nanoparticles).
A solid that is broken down into a powder
has the largest
surface area
and therefore has the fastest reaction rate.
This is why a catalyst is often used in the form of a powder.
In the
reaction between
calcium carbonate
and dilute hydrochloric
acid, calcium carbonate
may be used in the form of marble chips.
HCl + calcium carbonate
![]() calcium chloride + carbon
dioxide + water.
calcium chloride + carbon
dioxide + water.
2HCl(aq) + CaCO3(s) ![]() CaCl2(aq) +
CO2(g) +
H2O(l)
CaCl2(aq) +
CO2(g) +
H2O(l)
The reaction rates of both large marble
chips and
small
marble chips can be compared - see below.
A fair test
must use the same mass
of marble chips
for each reaction. The reaction
rate can be
followed by plotting the loss of mass against
time.
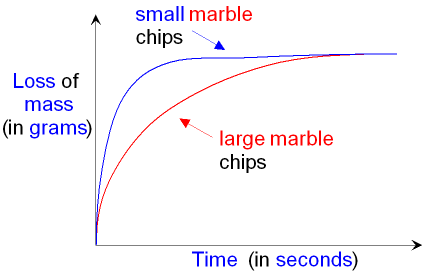
The reaction rate is
faster (the
slope is steeper)
for the reaction with the small marble
chips because
these have a greater surface area and
therefore they produce carbon dioxide more
quickly.
Both lines on the graph
finish at the same place. This
shows that the final loss of mass (the amount of carbon
dioxide produced) is the same for both reactions.
This is because the same mass of calcium
carbonate
(marble chips)
will produce the same mass of
carbon dioxide whether the chips are
large or small.
The smaller chips will just do it more
quickly.
![]() Links
Catalysts and Energy
Enzymes
Revision Questions
Links
Catalysts and Energy
Enzymes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Reaction Rate Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.