The structure extends (repeats itself) in all directions
giving a crystal with a regular arrangement of ions called
a lattice. The crystal is said to be highly ordered.
gcsescience.com 15 gcsescience.com
What is the Structure of an Ionic Compound?
What is a Crystal?
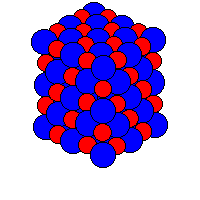
Below is a picture
of a small piece of a giant ionic structure.
It forms a crystal. It is also called a giant ionic
lattice.
The word "giant" means that a
huge number of particles
are present (compare this with the word simple).
A giant ionic structure
is made from a huge number
of ions.
In the picture
below the red balls are positive
ions and the
blue balls are
negative
ions.
The electrostatic
attraction
between the
oppositely
charged
ions is called an ionic bond
(compare this with a covalent
bond).

The structure
extends (repeats
itself) in all directions
giving a crystal with a regular arrangement of ions called
a lattice.
The crystal is said to be highly ordered.
For sodium chloride
the red balls may represent
Na+ ions
and the blue balls may represent
Cl- ions.
All ionic
solids have a similar
structure
to that
shown above but they may not be
identical.
Differences of packing and the relative
size and
amounts
of
ions
will give different crystal variations.
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Ionic Bonding Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.