gcsescience.com 7 gcsescience.com
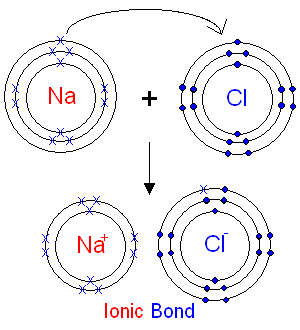
The Reaction between Sodium and Chlorine.
Chlorine has 7
electrons in its outer shell.
It is in group 7 of the
periodic table.
When an atom of chlorine
reacts
it will gain one electron from
sodium. The outer shell of chlorine will then have 8 electrons
and be full.
The chloride ion will have an extra electron
and
therefore an extra negative charge (shown as a - sign).

What is an Ionic Bond?
The electrostatic
force of attraction
between
the
oppositely
charged ions is called an ionic bond.
The electrons are shown as dots and crosses
to
show how they have moved during the reaction.
In reality all electrons are
identical.
The balanced chemical equation for the above reaction is
sodium + chlorine ![]() sodium chloride.
sodium chloride.
2Na(s)
+ Cl2(g)
![]() 2NaCl(s)
2NaCl(s)
Sodium chloride is a crystalline
ionic compound.
It forms a giant structure.
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Ionic Bonding Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.