gcsescience.com 6 gcsescience.com
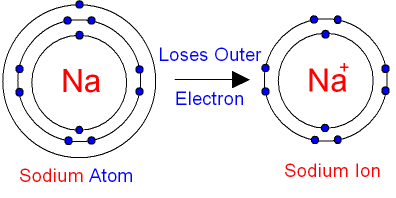
What happens when a Sodium Atom becomes a Sodium Ion?
A sodium
atom has 1
electron
in its outer shell.
It is in group 1 of the
periodic table.
When sodium reacts with non-metals
(for example chlorine) it will
lose its outer
electron. Its outer shell will
then have no electrons.
It is as though the outer shell has
vanished. The next shell in is full.
This full inner
shell becomes the new full outer
shell.

The sodium atom
loses its outer
electron to become a sodium
ion.
The sodium ion still has 11 protons (11 positive charges)
but now only 10 electrons
(10 negative charges).
The sodium ion has an extra positive charge, shown by the + sign.
All group 1
metals will form a 1+ ion
when they react with
non-metals.
The charge on the ion can also be shown as
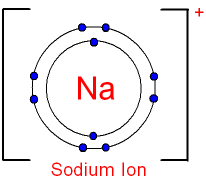
and the electron
structure written as [2,
8]+
The charge on the sodium ion will make it react
and form
ionic
bonds
with other oppositely
charged ions.
The full outer shell
of electrons
does not make the sodium ion
unreactive (see an example).
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Ionic Bonding Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.