
gcsescience.com 1 gcsescience.com
What is an Atom?
An atom is the smallest piece of an element
that can
exist.
Everything
is made of atoms.
Atoms are very small.
7 million atoms joined together
in a
straight line
would be about
1mm long.
What is the Structure
of an Atom?
All
atoms have a nucleus (the big
bit in the middle).
The nucleus contains protons and neutrons.
All
atoms have electrons.
The electrons are in shells
around the nucleus.
For any atom, the number of protons
is
the
same as the number of electrons.
If an atom loses or gains
electrons it is called
an ion.
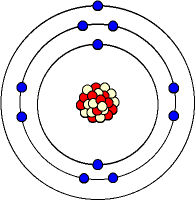
Below is a picture of a
sodium atom.
It has 11 protons, 11 electrons and 12 neutrons.


The electron structure is 2, 8, 1.
Each proton has an electrical
charge of +1.
Each electron has an electrical charge
of -1.
The neutron has no charge (it is
neutral).
An atom has the same number of protons and electrons
so the overall charge is zero (it is neutral).
The mass of a neutron and
a proton are the
same.
An electron is very much smaller,
about 1 ÷ 2000
times the size of a proton
although it has an equal and opposite electrical charge.
The electrons, although tiny, take up most of the
space of an atom.
This means that most of the
space that an atom fills
contains hardly any
mass. An atom is mostly empty space
with nearly all the
mass centred at the nucleus.
Summary
| Particle | Relative Mass | Relative Charge |
| Proton | 1 | +1 |
| Neutron | 1 | 0 |
| Electron | 0·0005 | -1 |
The protons, neutrons and
electrons are shown as
coloured to
distinguish them from each
other. In reality they have no colour.
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Atomic Structure Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.