gcsescience.com 4 gcsescience.com
What are Electron Shells (energy levels)?
Electrons in atoms are
in
shells
(shown as circles around the
nucleus).
The shells can also be called
energy levels.
We will use the term shell rather than
energy level
but either is acceptable.
The maximum number of
electrons in each shell,
going from the middle to the
outside, is 2, 8, 8, 18.
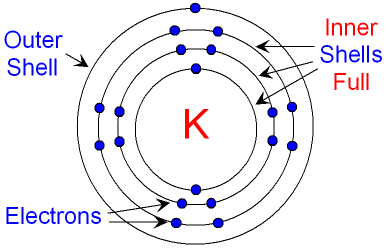
Below is a picture of a potassium
atom.
Its electron structure is
2, 8,
8, 1.

An atom that has the maximum number of
electrons
in its outer shell
will be stable. This means that
it will not
react with other atoms.
It belongs to a group called the
noble
gases. When the outer shell
has the maximum number of
electrons, the
electron shells
are said to be
full. The inner shells of an
atom are always
full.
If the outer
shell of an atom has
less than
its maximum number of
electrons
(see potassium above) then it will not be stable.
It will react
with other atoms to get a full
outer shell.
Ions
also
have a full outer shell of electrons
but
because they have a charge they will react
and
form ionic
bonds
with other oppositely
charged ions.
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Atomic Structure Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.