gcsescience.com 35 gcsescience.com
Rutherford and Marsden's Scattering
Experiment.
Continued from the previous page.
What were the Conclusions
from Rutherford and Marsden's Scattering
Experiment?
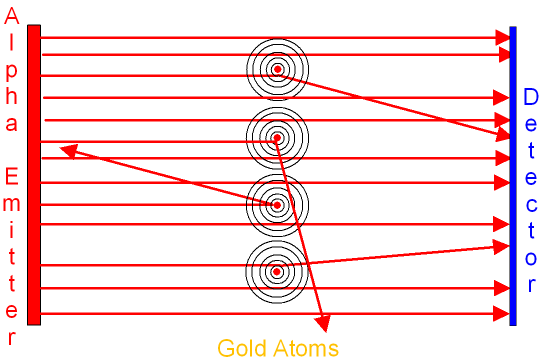
From the results of the scattering experiment on gold
foil (see below)
Rutherford and Marsden drew the following conclusions.
1. Since most of the alpha particles went
straight through the foil
most
of the space taken up by the atoms must be empty.
2. Since some of the positively charged alpha particles
were scattered back towards the
emitter, they must
have been repelled by a positive part of the atom (the nucleus).
3. Since the alpha
particles were
very fast moving,
they have a relatively large
momentum.
The positive nucleus of the gold atom
must have a large mass
to be able to stop some of the alpha particles
from moving forward and then repel
them back again.

In the above picture, the black circles represent the
electron shells.
The alpha particles travel straight
through the
electron shells without changing direction.
The red
circles represent the positive
nucleus.
If the alpha particle gets close
to the positive nucleus
it is repelled and changes its direction.
The closer the alpha particle gets
to the positive nucleus
the more it changes its direction.
If the alpha particle goes straight towards the positive nucleus
it is repelled back towards the emitter. This accounts
for the scattering of
the alpha particles from the gold foil.
Rutherford and Marsden's model of the structure of the atom
has a small
positively charged nucleus which contains
nearly all of the mass and
electrons in
shells which have
almost no mass but take up most of the space.
This is the model of atomic structure which we use today.
![]() Links
Radioactivity
Revision Questions
Links
Radioactivity
Revision Questions
![]()
gcsescience.com Physics Quiz Index Radioactivity Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.