gcsescience.com 12 gcsescience.com
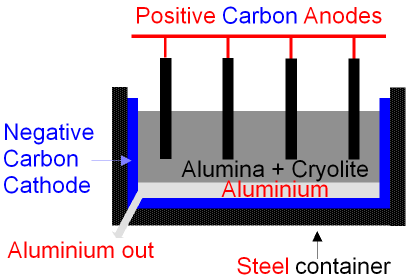
Extraction of Aluminium (continued) - Electrolysis Cell.

The steel container is coated
with carbon (graphite)
and this is used as the negative
electrode (cathode).
Aluminium oxide
(Al2O3) is an ionic compound.
When it is melted the Al3+ and O2- ions
are free to move
and
conduct electricity.
Electrolysis of the alumina/cryolite
solution
gives aluminium at the cathode and oxygen at
the anode.
4Al3+ + 12e- ![]() 4Al (aluminium metal at the (-)cathode) reduction.
4Al (aluminium metal at the (-)cathode) reduction.
6O2- -
12e-
![]() 3O2 (oxygen gas at the (+)anode) oxidation.
3O2 (oxygen gas at the (+)anode) oxidation.
Aluminium is more
dense than the
alumina/cryolite solution
and so it falls to the bottom of the cell
where it can be tapped off as
pure liquid
metal.
The overall reaction is
aluminium oxide ![]() aluminium + oxygen.
aluminium + oxygen.
2Al2O3(l)
![]() 4Al(l)
+ 3O2(g)
4Al(l)
+ 3O2(g)
Oxygen is given
off at the positive carbon
anode.
Carbon dioxide is also
given off
at the carbon anode because
hot oxygen reacts with
the carbon anode to form carbon
dioxide gas.
carbon + oxygen ![]() carbon dioxide.
carbon dioxide.
C(s) +
O2(g) ![]() CO2(g)
CO2(g)
The carbon anodes slowly
disappear
because each molecule of carbon
dioxide which is given
off
takes a little piece of carbon away with it.
The carbon anodes need to be replaced when they become too small.
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Metal Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.