gcsescience.com 3 gcsescience.com
What is Electrolysis?
Electrolysis is
the process where an electric
current is
passed through a liquid that conducts
electricity.
A liquid will only conduct
electricity if it contains ions.

The electrodes are often made from graphite.
The liquid that conducts electricity is called the electrolyte.
The amount of electricity needed to produce a
particular mass of metal (or non-metal) can be calculated.
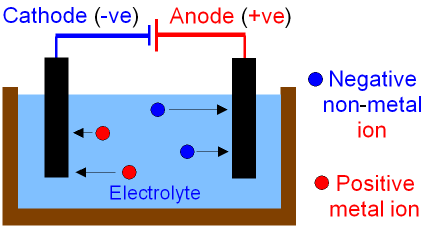
The negative
electrode, called the cathode,
will attract positively charged metal
ions.
The metal ions collect electrons from the cathode
(this is called reduction)
and are discharged as metal atoms.
The positive electrode,
called the anode,
will attract negatively charged non-metal ions.
The non-metal ions lose electrons
to
the anode (this is called oxidation)
and
are discharged as non-metal
atoms
which often combine
to form molecules.
In this way, elements that are present in
ionic compounds
can be separated by electrolysis. This method is
used for the extraction of
some metals from their ore.
See for example lead bromide, magnesium chloride,
potassium
chloride, sodium chloride and zinc chloride.
The situation is more complicated
when the substance
used for electrolysis is dissolved
in water.
Electrolysis can also be used for
metal plating.
![]() Links
Electrolysis
Revision Questions
Links
Electrolysis
Revision Questions
![]()
gcsescience.com The Periodic Table Index Metal Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.