gcsescience.com 4 gcsescience.com
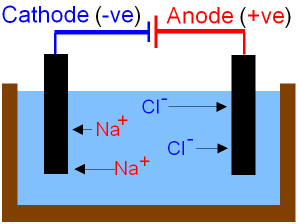
Electrolysis of Sodium Chloride.
This page shows the electrolysis of pure sodium
chloride.
You get different products
if the
sodium chloride is dissolved in
water.
Sodium
chloride must be
heated until it is molten
before it
will conduct electricity.
Electrolysis
separates the
molten ionic compound into its
elements.

What is a Half Equation?
The reactions at each electrode are called half
equations.
The half equations are written so that
the
same number of electrons occur in each equation.
2Na+ + 2e- ![]() 2Na (sodium
metal at the (-)cathode).
2Na (sodium
metal at the (-)cathode).
2Cl- -
2e-
![]() Cl2
(chlorine gas at the (+)anode).
Cl2
(chlorine gas at the (+)anode).
Sodium
ions
gain
electrons (reduction) to form
sodium atoms.
Chloride ions lose
electrons (oxidation) to form
chlorine atoms.
The chlorine atoms combine to form molecules of
chlorine gas.
The overall reaction is
2Na+Cl-(l)
![]() 2Na(s)
+ Cl2(g)
2Na(s)
+ Cl2(g)
Sodium can be used
in street lamps
and as a coolant in some
nuclear reactors. See some other examples of
electrolysis.
![]() Links
Electrolysis
Revision Questions
Links
Electrolysis
Revision Questions
![]()
gcsescience.com The Periodic Table Index Metal Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.