gcsescience.com 12 gcsescience.com
What is an Aqueous Solution?
When a substance
is
dissolved
in water it is called an
aqueous
solution.
What are the
Products
of the Electrolysis of Aqueous Solutions?
The electrolysis
of an aqueous solution
can
give products from the ions
in water rather
than the
dissolved substance. Water
is a compound
made from the elements
hydrogen and oxygen.
We can predict which products
will be discharged from
the electrodes by looking at the reactivity series.
If the metal
is above
hydrogen in the reactivity
series then
hydrogen
gas is discharged at the cathode.
If the
metal is below hydrogen
then the metal is discharged.
The table below shows the results
of the electrolysis
of some substances that are dissolved in water.
| Dissolved Substance | Formula | Cathode Product | Anode Product |
| Copper Chloride | CuCl2(aq) | Copper | Chlorine Cl2(g) |
| Copper Sulfate | CuSO4(aq) | Copper | Oxygen O2(g) |
| Sodium Chloride | NaCl(aq) | Hydrogen H2(g) | Chlorine Cl2(g) |
| Sodium Sulfate | Na2SO4(aq) | Hydrogen H2(g) | Oxygen O2(g) |
| Hydrochloric Acid | HCl(aq) | Hydrogen H2(g) | Chlorine Cl2(g) |
| Sulfuric Acid | H2SO4(aq) | Hydrogen H2(g) | Oxygen O2(g) |
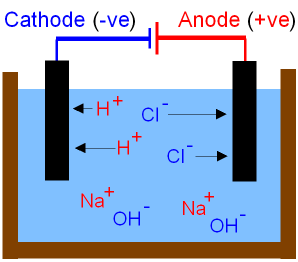
The Electrolysis of Sodium Chloride in
Water.
See the previous
page
for more detail of the electrolysis of sodium
chloride in water.

Question.
If water is a covalent
molecule, where have the
H+ hydrogen
ions
and OH-
hydroxide ions
come from in the picture above?
Answer.
The ionisation of water
makes hydrogen
ions and hydroxide ions.
Although hydrogen ions are only
present in small amounts the
reversible
ionisation means that the hydrogen ions
are instantly
replaced
when they are discharged at the
cathode as hydrogen gas.
The water never runs out of hydrogen
ions and hydroxide ions.
Question.
Why don't the Na+ sodium ions and hydroxide ions
get discharged at the electrodes?
Answer.
The (-)cathode attracts both Na+
sodium ions and
H+ hydrogen ions.
Only one type of ion at a time can be discharged at the electrode.
The ion that is lower in the reactivity series is discharged first.
Hydrogen gas is discharged leaving the sodium ions in solution.
The (+)anode attracts both Cl- chloride
ions and OH-
hydroxide ions.
Chlorine gas is discharged leaving the hydroxide ions in
solution.
The solution
contains both sodium ions and hydroxide ions
and therefore becomes sodium
hydroxide(aq).
See the uses of hydrogen,
the uses
of chlorine and also the uses
of sodium hydroxide.
![]() Links
Alkali Metals
Revision Questions
Links
Alkali Metals
Revision Questions
![]()
gcsescience.com The Periodic Table Index Periodic Table Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.