gcsescience.com 29 gcsescience.com
What is an Alloy?
An alloy is a mixture of
metals that has
different (improved)
properties from the metal elements that make
it.
The pure metal is usually too
soft to be useful. The figures
given for percentages of elements in an alloy
are for guidance only,
as any particular alloy may be designed
for a particular use.
Often an alloy is composed mainly of one metal
(the parent metal)
with small amounts of other metals added. The other metals
replace positions of the parent metal in the metal
structure,
or sometimes fit into the spaces in
the metal structure.

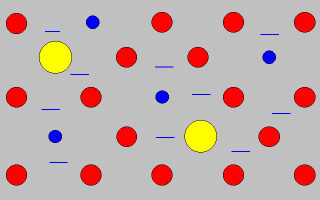
The red
balls are the parent metal.
The
blue and yellow
balls are other metals.
The blue lines represent free electrons.
Compare this with the metal structure of a
pure metal.
The other
metals in the structure can
change the properties of
the alloy
by preventing the metal ions from sliding over each
other.
This can make the alloy tougher and stronger
than the parent metal.
![]() Links
Alloys
Revision Quizzes
Revision Questions
Links
Alloys
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Metal Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.