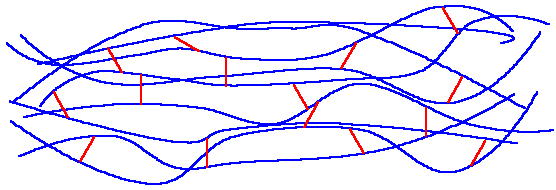
for a thermosetting polymer. The red lines
represent the cross links between the chains.
gcsescience.com 61 gcsescience.com
The Structure of Polymers. What are Cross Links?
Polymers may be classified as thermosoftening or thermosetting.
What is a Thermosetting Polymer?
Thermosetting polymers have their chains cross linked
by
covalent
bonds. The starting materials are placed into
a mould to form the desired
shape. The polymer is then
heated (or initiated with
uv light) and chemical
reactions
occur to form the cross
links between the chains.
The resulting three dimensional
solid structure cannot then be
changed.
Further heating will not cause the polymer
to soften,
melt
or change shape (unlike thermosoftening polymers).
Examples of thermosetting polymers are
1. Melamine resin - used in furniture.
2. Bakelite - used for
saucepan handles
and electric light
fittings.
3. Epoxy resins - used in many glues.
The picture below shows a
typical structure
for a thermosetting polymer.
The red lines
represent the
cross links between the chains.

![]() Links Polymers Revision Questions
Links Polymers Revision Questions ![]()
gcsescience.com The Periodic Table Index Polymers Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.