gcsescience.com 37 gcsescience.com
What is the Structure of Graphite?
When you come
across carbon as a reactant or electrode,
carbon means graphite not diamond.
It can be written C(gr) but
is usually written as just C.
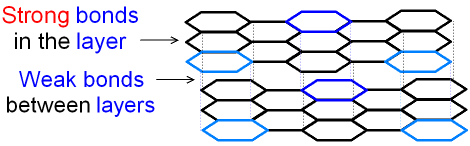
The structure of graphite consists of many flat layers
of hexagons. The layers are called graphene sheets.
Each carbon atom in the layer is joined
by strong
covalent
bonds to only three other carbon atoms.
Compare this with the structure of diamond.
Each graphene sheet is itself a giant molecule.
Carbon is in group 4 of the periodic table and so it has
four electrons in its outer shell.
Three of these electrons
are used for covalent bonding in the graphene sheet.

What are the Properties of Graphite?
There are no
covalent bonds between the layers
and so
the layers can easily
slide over each other making graphite
soft and slippery and an
excellent lubricant (like oil).
The fourth electron between the
layers is delocalised.
It is a
free
electron and these free electrons between
the
layers allows graphite to conduct
electricity and heat.
See also carbon fibres and fullerenes.
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Covalent Bonding Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.