gcsescience.com 9 gcsescience.com
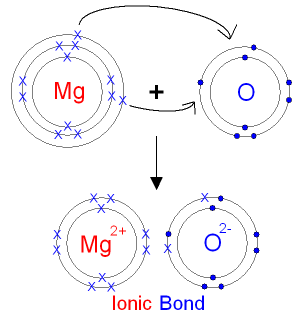
The Reaction between Magnesium and Oxygen.
Magnesium is in group 2 of the periodic table.
A magnesium atom will lose 2 electrons to form
a stable
2+ ion.
Oxygen is in group 6
of the periodic table.
An oxygen atom will gain
2 electrons to form a stable 2- ion.
In this example the electrons are shown as
dots and
crosses.
You will often see electrons drawn
like this in books.

The ionic
bond between magnesium and oxygen
is stronger
than the ionic bond between sodium and
chlorine
because of the greater charge on the ions.
Magnesium oxide has a higher melting
point
because of the stronger bond.
magnesium + oxygen ![]() magnesium oxide.
magnesium oxide.
2Mg(s) + O2(g) ![]() 2MgO(s)
2MgO(s)
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Ionic Bonding Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.