the magnesium atom. The atoms become ions.
gcsescience.com 11 gcsescience.com
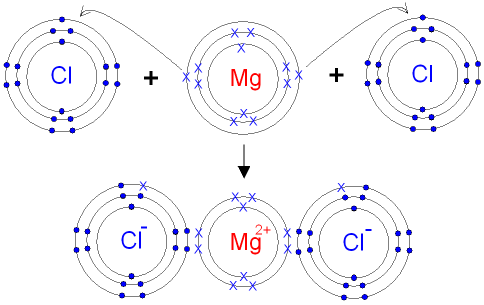
The Ionic Bond formation for Magnesium Chloride.
Magnesium is in group 2 of the periodic table.
A magnesium atom will lose 2 electrons to form
a stable
2+ ion.
Chlorine
is in group 7 of the
periodic table.
A chlorine atom will gain
1 electron to form a stable 1- ion.
In this example the electrons are shown as dots and crosses.
Two chlorine atoms will
each gain one
electron from
the magnesium
atom. The atoms become ions.

The attraction between the oppositely charged
ions
forms the ionic bond between magnesium and chlorine.
The formula for
magnesium chloride is MgCl2.
magnesium +
chlorine ![]() magnesium chloride.
magnesium chloride.
Mg(s) + Cl2(g) ![]() MgCl2(s)
MgCl2(s)
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Ionic Bonding Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.