gcsescience.com 34 gcsescience.com
Using the Amount of Heat to find the End Point of a Titration.
The end point can be found
by measuring the amount of
heat
released into the
solution.
The reaction between an acid and an
alkali is
exothermic.
If a thermometer is placed in the
solution in the conical
flask
the temperature will
increase when acid is added to the alkali.
At neutralisation the amount of heat
released will decrease
because
there are no more
hydroxide
ions left for the hydrogen ions to react with.
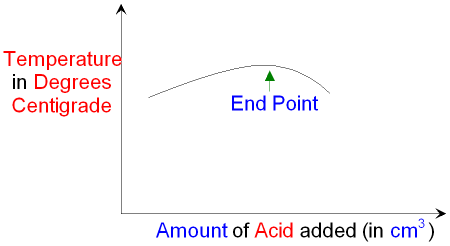
If you draw a graph of temperature against the amount of
acid
you can see where the end point is.

The titration can be repeated
with the same amounts of acid and alkali used
at the end point.
Pure salt crystals can then be crystallised from the neutral solution.
This is the least accurate of the 4 methods
because heat is lost
from
the conical flask to the environment during the titration.
It may not be very obvious where the end point occurs.
![]() Links
Acids
and Alkalis
Revision Questions
Links
Acids
and Alkalis
Revision Questions
![]()
gcsescience.com The Periodic Table Index Neutralisation Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.