gcsescience.com 24 gcsescience.com
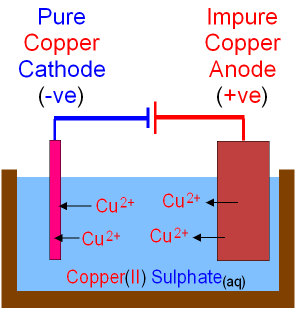
Extraction of Copper - Purification by Electrolysis.
High purity copper is needed to make electrical wires.

The anode is a block of impure copper.
The cathode is a thin piece of
pure copper.
The electrolyte is copper
sulfate.
When electricity is passed through the cell
copper is dissolved at the anode by oxidation
and Cu2+ ions go into solution.
Cu(s) - 2e- ![]() Cu2+(aq)
Cu2+(aq)
At the cathode, copper is deposited by reduction.
Cu2+(aq) +
2e-
![]() Cu(s)
Cu(s)
As copper
ions move from the anode to the
cathode
the anode gets smaller as the cathode gets bigger.
This is a redox
reaction (continued).
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Metal Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.