4Fe(s) + 3O2(g)
gcsescience.com 33 gcsescience.com
Corrosion (rusting) of Iron and Steel.
Iron exposed to moist
air will react
slowly
with
oxygen in the air to form iron
oxide.
This oxidation
process is called rusting.
iron + oxygen
![]() iron(III)
oxide.
iron(III)
oxide.
4Fe(s)
+ 3O2(g)
![]() 2Fe2O3(s)
2Fe2O3(s)
Rusting requires both oxygen and water.
Salt or acid will accelerate
rusting.
Rusting may be prevented by
1) coating the surface (by painting for example)
to prevent
contact with the air.
2) sacrificial
protection of a more
reactive metal.
Iron
and steel are most commonly protected by
painting (for example bridges, buildings,
vehicles),
metal plating (see below)
or plastic coating (for example household
equipment).
Metal Plating.
Zinc plating is called galvanizing (this is also sacrificial
protection).
Chromium plating is used on water taps and
some
car wheels to give a highly polished protective
surface.
The metal plating process
(also called electroplating)
uses electrolysis of a solution containing ions
of the plating metal.
The anode is
made from the pure plating metal.
The metal object that needs plating is used as the cathode.
Most metals can be plated.
Common plating metals are gold,
nickel and silver
as well as chromium and zinc referred to above.
Silver plating could be done in the cell below.
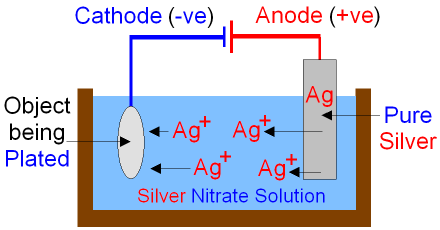
When electricity is passed through the cell
silver is dissolved at the anode by oxidation.
Ag+ ions
go into the
silver nitrate
solution.
Ag(s) -
e-
![]() Ag+(aq)
Ag+(aq)
Silver is deposited
onto the
surface
of the object by reduction at the cathode.
Ag+(aq) +
e-
![]() Ag(s)
Ag(s)
As silver
ions move from the anode to the
cathode
the anode gets smaller as the object becomes silver plated.
This is a redox
reaction.
The rate at which the silver
ions enter the electrolyte from the
anode is the same as the rate at
which the silver ions
leave the
electrolyte at the cathode. The concentration of
the silver nitrate solution
therefore remains unchanged.
![]() Links
Alloys
Revision Quizzes
Revision Questions
Links
Alloys
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Metal Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.