their 1 electron with carbon
to form six carbon - hydrogen covalent bonds.
The two carbon atoms will each share
their 1 electron to form one carbon - carbon
covalent bond and make an ethane molecule (C2H6).
gcsescience.com 10 gcsescience.com
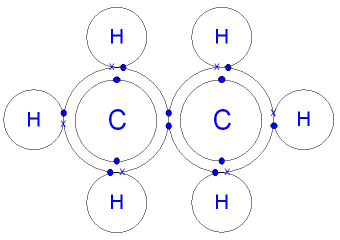
What is the Structure of an Ethane Molecule?
A carbon atom has 4
electrons in its
outer
shell.
Carbon is in group 4 of the periodic table.
A hydrogen atom has 1 electron in its
outer shell.
Hydrogen can only form 1 bond.
The six hydrogen atoms will
each share
their 1 electron with carbon
to form six carbon - hydrogen covalent bonds.
The two carbon atoms will each share
their 1 electron to form
one carbon - carbon
covalent bond and make an ethane molecule (C2H6).

The pairs of shared
electrons between the
hydrogen
and the carbon atoms
show single covalent bonds - see covalent
bonding.
Ethane is commonly represented by the structural formula
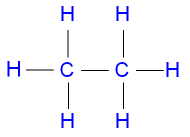
where the single lines between
the atoms
represent single covalent
bonds.
![]() Links Hydrocarbons Revision Questions
Links Hydrocarbons Revision Questions ![]()
gcsescience.com The Periodic Table Index Hydrocarbons Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.