gcsescience.com 34 gcsescience.com
Rutherford and Marsden's Scattering Experiment.
What is the Structure of an Atom?
In the past it was suggested that
an atom was a large
area
of positive
charge with negative electrons stuck in
it.
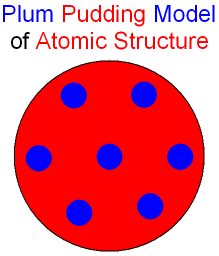
This was called the plum pudding model where
the electrons
(shown in the picture below as blue balls)
were like plums stuck in a
positive pudding (the big red ball).

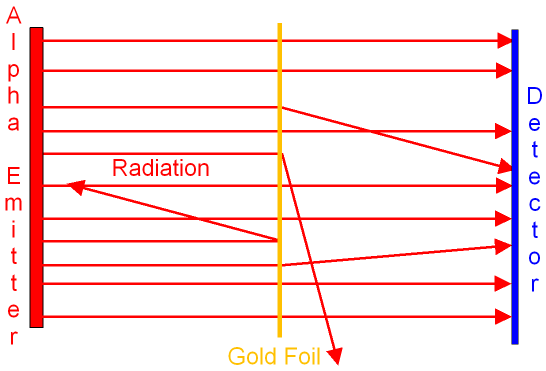
Rutherford and
Marsden fired very fast alpha particles
into a very thin piece of gold
sheet (called gold foil).
The foil was only a few atoms thick and most of the
alpha particles
went straight through it to the detector.
When the detector was moved around the
foil they
were surprised to find
that a small number of alpha particles
seemed to have been scattered in all
directions.
Some of the alpha particles even came
back towards the emitter.
Rutherford and Marsden suggested a structure for the atom
which would account for the
scattering of these alpha particles.
![]() Links
Radioactivity
Revision Questions
Links
Radioactivity
Revision Questions
![]()
gcsescience.com Physics Quiz Index Radioactivity Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.