gcsescience.com 19 gcsescience.com
Elements, Compounds and Mixtures
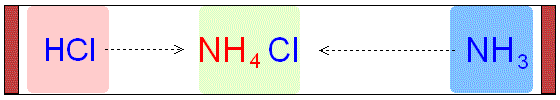
The Diffusion of Hydrogen
Chloride and Ammonia Gas
through Air to form Ammonium
Chloride.
Cotton
wool soaked in concentrated ammonia
solution, NH3(aq)
and concentrated hydrogen
chloride
solution
(also called hydrochloric
acid) HCl(aq)
are placed at each end of a sealed
tube.
The cotton wool with ammonia solution gives off
ammonia molecules (NH3).
The cotton wool with hydrochloric acid gives off
hydrogen chloride molecules
(HCl).

HCl
and NH3 molecules diffuse through the
air towards each other.
When they meet, they react to form a white powder
called ammonium chloride, NH4Cl.
hydrogen
chloride +
ammonia ![]() ammonium chloride.
ammonium chloride.
HCl(g)
+ NH3(g) ![]() NH4Cl(s)
NH4Cl(s)
The ![]() sign shows that the reaction is reversible.
sign shows that the reaction is reversible.
The ring of white powder is closer to
the HCl than the NH3.
This is because the NH3
molecules are lighter (smaller)
and have diffused more quickly through
the air in the tube
(you can work out which molecule is
lighter by looking at the RFM).
Note that lighter (smaller)
particles move more quickly
than heavier (larger) ones at the same temperature.
![]() Links
Solid
Liquid
Gas
Revision Questions
Links
Solid
Liquid
Gas
Revision Questions
![]()
gcsescience.com The Periodic Table Index Chemistry Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.