gcsescience.com 10 gcsescience.com
Elements, Compounds and Mixtures
What is Paper Chromatography?
Paper Chromatography
is a separation
technique
that is used to separate and identify
the
components of a mixture
(see also gas
chromatography).
Paper
chromatography is used to identify colouring
agents
(chemicals) for example in food
or ink.
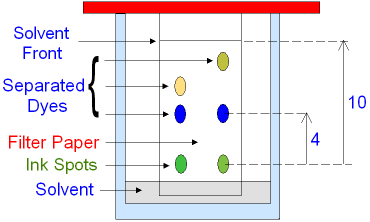
The mixture (in
this case two green ink spots)
is put on a filter paper that is
placed in a suitable solvent.

As the solvent rises up the filter paper
the individual components (dyes)
within the green ink spots
are separated.
Different dyes travel different
distances up the paper.
The solvent travels furthest
up the filter
paper leaving a line called the solvent front.
What is the Rf value?
The distance travelled up the paper by a component
divided by the distance travelled up the paper by the solvent
is called the Rf value or retention
factor.
For example, if a component
travelled 4 cm
and the solvent
travelled 10 cm
then Rf = 4
÷ 10
= 0·4
In the above example the
green ink spots each have
the
same blue
dye
because they have
travelled the
same distance (same Rf value)
but different yellow dyes because they have
travelled a
different distance (different Rf value).
Paper
chromatography is used by
the food
industry and forensic
science.
![]() Links
Solid
Liquid
Gas
Revision Questions
Links
Solid
Liquid
Gas
Revision Questions
![]()
gcsescience.com The Periodic Table Index Chemistry Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.