gcsescience.com 12 gcsescience.com
What is the Structural Formula of Pentane and Hexane?
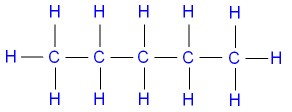
Pentane (C5H12) is commonly represented
by the structural formula
below (see
isomers)

and hexane
(C6H14) by the
structural formula
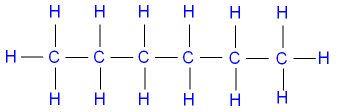
where the single lines between
the atoms
represent single covalent bonds.
Note that each carbon atom has
four bonds
(valency 4),
and each hydrogen atom has one bond
(valency 1).
Valency is the combining power of an atom.
![]() Links Hydrocarbons Revision Questions
Links Hydrocarbons Revision Questions
![]()
gcsescience.com The Periodic Table Index Hydrocarbons Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.