CH3OH(aq) + HCO2H(aq)
gcsescience.com 51 gcsescience.com
The Reaction of Methanoic Acid with Alcohols to make Esters.
Methanoic
acid will react with
alcohols in the presence of
concentrated sulfuric
acid, to form esters.
Concentrated sulfuric acid is a catalyst for this
reaction.
methanol + methanoic acid ![]() methyl methanoate + water.
methyl methanoate + water.
CH3OH(aq) + HCO2H(aq)
![]() HCO2CH3(aq)
+ H2O(l)
HCO2CH3(aq)
+ H2O(l)
The structure of methyl
methanoate.
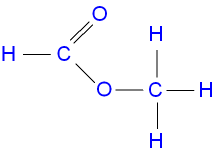
ethanol + methanoic acid ![]() ethyl methanoate + water.
ethyl methanoate + water.
C2H5OH(aq) + HCO2H(aq)
![]() HCO2C2H5(aq) + H2O(l)
HCO2C2H5(aq) + H2O(l)
The structure of ethyl
methanoate.
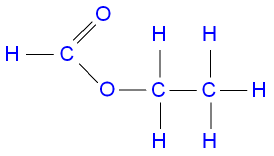
propanol + methanoic acid ![]() propyl methanoate + water.
propyl methanoate + water.
C3H7OH(aq) + HCO2H(aq)
![]() HCO2C3H7(aq) + H2O(l)
HCO2C3H7(aq) + H2O(l)
The structure of propyl
methanoate.
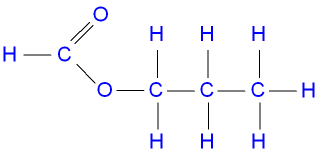
butanol + methanoic acid ![]() butyl methanoate + water.
butyl methanoate + water.
C4H9OH(aq) + HCO2H(aq) ![]() HCO2C4H9(aq)
+ H2O(l)
HCO2C4H9(aq)
+ H2O(l)
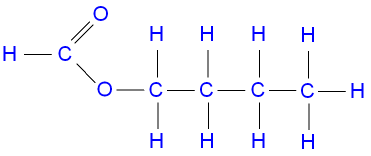
The structure of butyl
methanoate.
![]() Links Esters Revision Questions
Links Esters Revision Questions ![]()
gcsescience.com The Periodic Table Index Esters Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.