gcsescience.com 58 gcsescience.com
The Chemistry of the Polymerisation of Ethene.
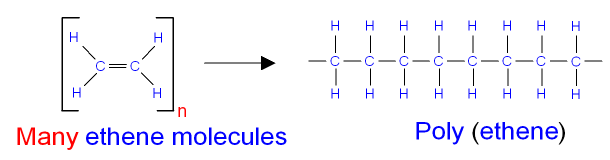
The picture below shows the polymerisation of ethene.

Ethene put under pressure and heated
with a catalyst
will polymerise
(make long chains of atoms)
to form poly(ethene).
Note that there are no double bonds in the polymer.
Poly(ethene) is an alkane. It is a saturated hydrocarbon.
Depending on the reaction
conditions and the type of catalyst used,
ethene can make either HDPE
or LDPE.
HDPE is High
Density Poly(Ethene)
and has a higher crystallinity
and a higher melting
point than LDPE
(Low Density Poly(Ethene).
HDPE is stronger
and stiffer
than LDPE.
What is an Addition Polymer?
A polymer that is formed from monomers added
together
where no other
substance is produced is called an
addition
polymer.
Examples of addition polymers
are
poly(ethene), poly(propene),
poly(tetrafluoroethene) - PTFE,
poly(chloroethene) - PVC and
poly(phenylethene) - polystyrene.
Chloroethene used to be called vinyl chloride.
The polymer is still called
polyvinyl chloride, or PVC.
Phenylethene used to be called styrene.
The polymer is still called
polystyrene.
What is the Repeat Unit?
A polymer is often written in the form shown below.
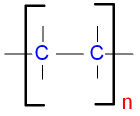
The brackets contain the repeat unit.
The small n means that there are many of them.
The repeat unit is repeated over and
over again
many times to make up
the long chain of the polymer.
Below are some examples of the
repeat units of polymers.
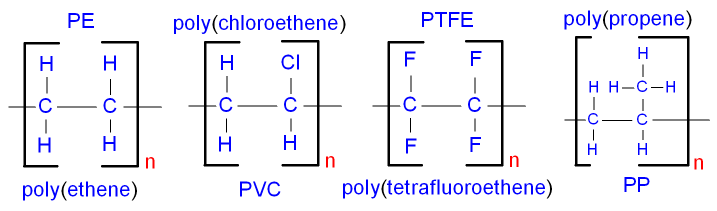
See the properties and uses of polymers.
![]() Links Polymers Revision Questions
Links Polymers Revision Questions ![]()
gcsescience.com The Periodic Table Index Polymers Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.