gcsescience.com 34 gcsescience.com
What are the Properties of Simple
Molecules?
(compare these with giant
molecules and ionic
compounds).
When non-metals react with non-metals
a covalently
bonded molecule is formed.
Covalent
bonds are strong but only exist
between the atoms of the molecule.
The force of attraction between molecules
(called the intermolecular force)
is very weak (compare this with ionic bonding).
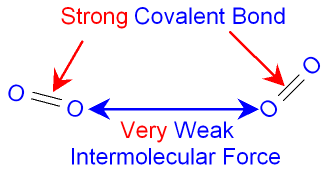
The
picture below
shows the
forces of attraction
between two
molecules of oxygen gas.

The weak
force between molecules
means that very
little energy
is needed to separate them. Many
molecular compounds
are
liquid or gas at room
temperature.
They have low melting and
boiling points.
Note that when a molecular compound melts or boils,
molecules separate from each other
but the covalent bond between the
atoms of the molecule
does not
break.
The molecule is the same molecule
in the solid, liquid or
gas state. If the covalent bond breaks,
the molecule
is said to decompose (split up!).
Molecules do not have
a charge (no ions) because
the
electrons are
shared between atoms to form a
covalent bond.
Molecular substances will therefore
not conduct electricity
(with the exception of graphite
which has an unusual structure).
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Covalent Bonding Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.