gcsescience.com 28 gcsescience.com
What is Calorimetry?
Measuring the change
in energy of a chemical
reaction
is called calorimetry. A simple method
for measuring
the amount of energy
given out by a fuel
is shown below.
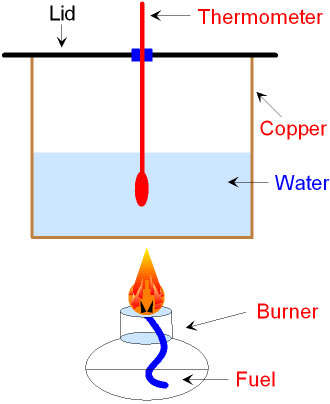
What is a Calorimeter?
The apparatus used to measure the change
in energy is called
a calorimeter.
A known amount of water is placed in a glass
or copper container.
Burning a fuel
is an exothermic
reaction.
The amount of energy released can be
calculated from
the
increase in temperature of the water
as the fuel burns.

The simple calorimeter
shown above
has some advantages and some disadvantages.
Disadvantages.
The calorimeter does not
collect all of the heat
released
from burning the fuel
because some heat is lost
to the
surroundings. The amount of heat lost
can be
minimised
by using a lid on top
of the calorimeter
and
by
putting a draught excluder all around it but some heat
is always lost and so this is not
a good method to
find the maximum amount of heat
available from a fuel.
Advantages.
The calorimeter is easy to make and use. The calorimeter
is made from copper which is an excellent conductor
of heat. The copper allows the heat
released from burning
the fuel to be conducted
efficiently to the water.
If the experiment is repeated
using different
fuels, the
amount of heat
lost by the calorimeter
is similar in
each case and so this is a good method for comparing
the amount of
heat released from different
fuels.
To make the comparison fair, the variables to be kept constant are
1. the amount of water in the calorimeter.
2. the starting temperature of the water.
3. the increase in temperature of the water.
4. the distance between the burner and the calorimeter.
The picture below shows a different type of
calorimeter
that can
be used for a chemical reaction
in a solution.
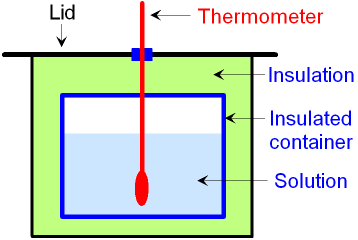
An insulated container
with a
thermometer inside is used to
measure the temperature change during the
reaction.
This type of calorimeter can be used for neutralisation
reactions.
![]() Links
Catalysts and Energy
Enzymes
Revision Questions
Links
Catalysts and Energy
Enzymes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Energy Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.