gcsescience.com 28 gcsescience.com
What is a Nitrogen Molecule?
Nitrogen is a non-metal.
A nitrogen atom has 5
electrons in its
outer
shell.
Nitrogen is in group 5 of the periodic table.
Two nitrogen atoms will each
share three
electrons to form
three
covalent bonds
and make a nitrogen molecule
(N2).
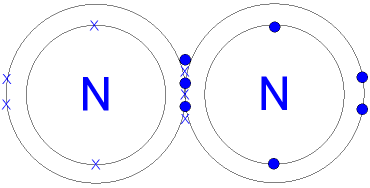
This is a picture of a nitrogen molecule.

By sharing the six
electrons where the shells touch
each nitrogen atom can count 8 electrons in its outer shell.
These full outer
shells with their shared electrons are now stable.
The N2 molecule will not
react further with other nitrogen
atoms.
Note the 3
pairs (6 electrons)
shared between the atoms.
Each electron
pair is one
bond.
Nitrogen has three
bonds between its atoms.
This is called a triple bond. The triple bond
is very strong
and this is what makes nitrogen so unreactive (stable).
The structural formula of an nitrogen molecule is written
![]()
There are no ions present (no + or - charges) in nitrogen
gas
because
the electrons are shared,
not
transferred from one atom to
another.
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Covalent Bonding Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.