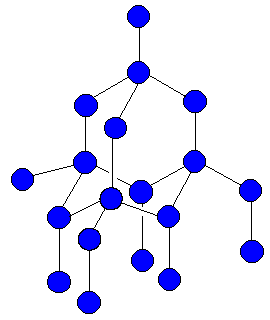
In the picture of diamond above, each blue ball represents
a carbon atom. The structure has billions
of atoms forming hexagons and extends in all directions.
gcsescience.com 36 gcsescience.com
What is the Structure of a Giant Molecule?
What is the Structure of Diamond and Silicon?
Diamond is a form of the element carbon - see also graphite.

In the picture
of diamond above, each
blue ball
represents
a carbon atom. The structure has billions
of atoms forming
hexagons and
extends in
all directions.
All
of the covalent
bonds in diamond are
identical.
It forms a
giant
molecular structure.
A crystal of diamond is one
giant molecule!
The covalent
bonds in diamond are very strong.
It is the hardest
naturally occurring material
and is used in machine cutting tools.
Diamond does not conduct
electricity (unlike graphite).
Silicon (used in microchips for computers) has the
same structure, with each
blue ball
being a silicon atom.
Silicon
dioxide (SiO2) is
sand (also called silica).
SiO2 has a similar structure and also
forms a giant molecule.
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Covalent Bonding Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.