gcsescience.com 41 gcsescience.com
Nanoscience and Nanoparticles.
What is Buckminsterfullerene?
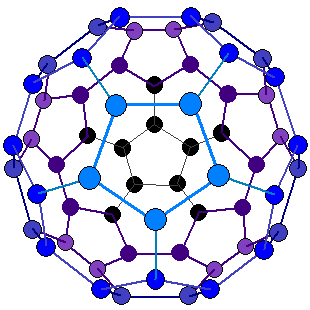
In 1985 a new allotrope of carbon (C60) was discovered.
Sixty carbon atoms form the shape of a
ball like a football
with a
carbon atom at each corner
of the 20
hexagons and 12
pentagons.
Each carbon atom (shown below as a
circle) has three bonds.

The size of the molecule is almost
exactly 1nm in
diameter.
The ratio of the size of an
ordinary soccer ball
to the planet
Earth
is the same as
the ratio of the size of a C60 molecule to a soccer
ball.
These are not called giant molecules
because there are only sixty
atoms.
A large number of these molecules can fit together
to form a transparent yellow
solid called fullerite.
This form of carbon
was named after the American architect
Buckminster Fuller,
who was famous for designing a large geodesic dome
which looked similar (sort of) to the molecular structure of C60.
Many other balls of carbon
called fullerenes,
have since been made, including C70, C76, and C84.
These molecules have become known
as "buckyballs".
Fullerenes are
used as catalysts
and lubricants.
They are also used in nanotubes for strengthening
materials
(for example sports equipment)
and are
sometimes used as a way of delivering drugs into the body.
![]() Links
Revision Quizzes
Revision Questions
Links
Revision Quizzes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Covalent Bonding Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.