gcsescience.com 71 gcsescience.com
Polymers - Smart Materials -
Hydrogels.
Wound Dressings and Drug Delivery.
What are the Uses of Hydrogels?
Hydrogels are used
to make soft contact lenses, nappies,
wound dressings and drug delivery
systems.
How is a Hydrogel used for a Wound Dressing?
A wound dressing is put over a
cut in the skin to help the skin
heal.
The hydrogel is applied as a thin
layer which is moist and soothing.
It stops the wound drying out and protects it from infection.
The hydrogel can control bleeding and does not
stick to the surface
so it can be removed
easily without damaging the
skin.
How is a Hydrogel used for Drug Delivery?
In drug delivery the
hydrogel can release an antibiotic (or other
drug)
at a controlled rate to the body
tissue beneath. This is better than
taking
an antibiotic as a pill by
mouth which has an effect on the
whole body
and increases the chance of a bad
reaction to the drug.
The
hydrogel is called a carrier when it is
loaded with a drug.
As the swelling of the
hydrogel increases,
the chains of the
cross
linked network move further apart
and the drug
can diffuse more
quickly through the hydrogel
to the skin.
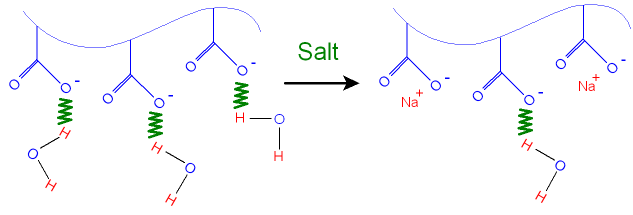
What is the Structure of a Hydrogel
with Salty Water?
We shall look at how the
hydrogel on the previous page
changes in response to an
increase in salt concentration.
The chains in the uncoiled cross
linked hydrogel
attract water
molecules by hydrogen bonding.
As more salt
(for example sodium chloride) is added
to the hydrogel, the positive sodium
ions take up places
next to the
negative oxide ions and there is less space
for the
water molecules as shown in the picture
below.

This makes the hydrogel lose some
water. The
negative
charges along the chain
repel
each other less in the
presence of the sodium ions
and so the chains become more
coiled up.
This also squeezes out
water from
the hydrogel.
The result is that a small
change in salt concentration
can have
a significant effect
on the amount of
water
leaving the
hydrogel.
![]() Links Polymers Revision Questions
Links Polymers Revision Questions ![]()
gcsescience.com The Periodic Table Index Polymers Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.