gcsescience.com 70 gcsescience.com
Polymers - Smart Materials -
Hydrogels.
Sodium Polyacrylate and Hydrogen Bonding.
What is a Hydrogel?
A hydrogel is an example of a smart material. It can
change its
structure in response to salt concentration, pH and temperature.
What is the Structure of a Hydrogel?
Hydrogels are cross
linked polymers that have
hydrophillic groups.
They are often polymers containing
carboxylic acid
groups. One
common polymer used to make
hydrogels is sodium polyacrylate.
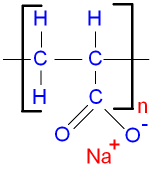
The chemical name for this polymer is poly(sodium propenoate).
The repeat unit for the structure is shown below.

The polymer chains usually exist
in the shape of
randomly
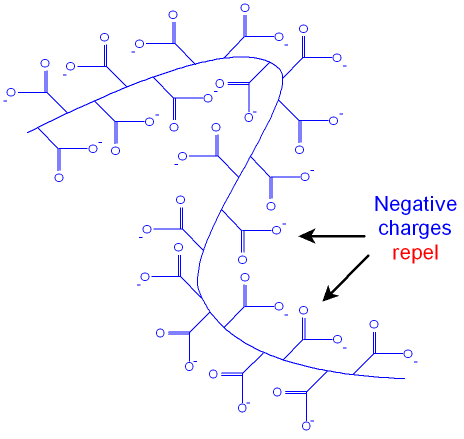
coiled molecules. In the absence of Na+
ions
(if you
remove all the the salt) the negative charges on the
oxide ions along the polymer chain all repel each
other
and the chains tend to uncoil as shown in the picture
below.
Water
molecules are
attracted
to the negative
charges by hydrogen
bonding.
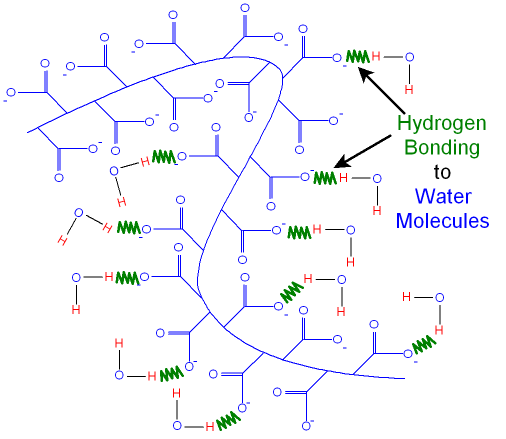
In this state the hydrogel can absorb over five hundred times
its own weight of
pure water but less salty water.
This ability to absorb so much water makes the hydrogel
useful
for the lining of babies' nappies -
see other
uses.
When salt is added to the
hydrogel, the chains start to
change
their shape and water is
lost from the gel (see the next page).
![]() Links Polymers Revision Questions
Links Polymers Revision Questions ![]()
gcsescience.com The Periodic Table Index Polymers Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.