gcsescience.com 64 gcsescience.com
Polymers - Poly(ethenol) - Slime and Hydrogen Bonding.
How is Slime Made?
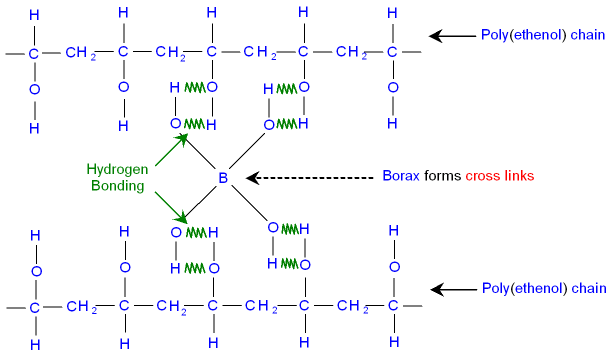
Poly(ethenol) will react with borax to make slime.
Borax
is sodium tetraborate - Na2B4O7.
When borax is put into water it forms B(OH)4- ions.
These ions interact with the
OH groups and make cross links
between the poly(ethenol) chains forming slime.
This type of interaction is called
hydrogen bonding, see below.
Using more borax gives a
greater number of cross
links
and a higher
viscosity of the slime.
Viscosity is a measure of how easily something flows.
Water has a low viscosity and thick syrup has a high
viscosity.
The picture below shows hydrogen bonding in slime.

What is Hydrogen Bonding?
There is an attraction between the H and the O because
the H has a small positive
charge and the O has a small
negative charge. The opposite
charges attract each
other
and this attraction is called hydrogen
bonding. In hydrogen
bonding the attraction between
the H and the O is
weaker
than the attraction in either an ionic
bond or a covalent
bond.
![]() Links Polymers Revision Questions
Links Polymers Revision Questions ![]()
gcsescience.com The Periodic Table Index Polymers Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.