gcsescience.com 25 gcsescience.com
What is pH?
pH is a measure of how acidic or how alkaline a solution is.
The pH of a solution
depends on the
degree of ionisation of an acid
or alkali
as well as how concentrated
or dilute it is.
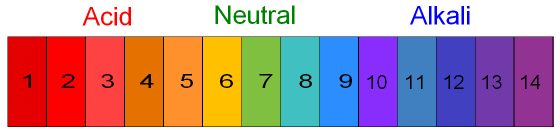
The pH scale goes from 1 to 14
with 1 being very strongly acidic
and 14 being very strongly alkaline.
A pH of 7
is neutral.
You can measure the pH of a
solution using universal indicator.
Just as litmus paper will be
red for an acid and blue for an
alkali,
so universal indicator is a
mixture of indicators
that will give a different colour for
a different pH.

Any acid will have a pH of less
than 7.
Any alkali will have a pH of more than
7.
Strong acids (HCl or H2SO4 or HNO3) have a pH of 1 (red).
Weak
acids have a pH of 3 to 4 (orange).
Examples of weak acids are ethanoic acid (vinegar),
citric acid
(lemon juice)
and rain water.
Rain water has a natural pH of 5·5 (see carbonic acid).
Water and salts are neutral. They have a pH of 7 (green).
A weak alkali (ammonia) has a pH of 11 to 12 (blue).
A strong alkali (NaOH) has a pH of 14 (purple).
![]() Links
Acids
and Alkalis
Revision Questions
Links
Acids
and Alkalis
Revision Questions
![]()
gcsescience.com The Periodic Table Index Neutralisation Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.