gcsescience.com 3 gcsescience.com
What is the Fractional Distillation of Crude Oil?
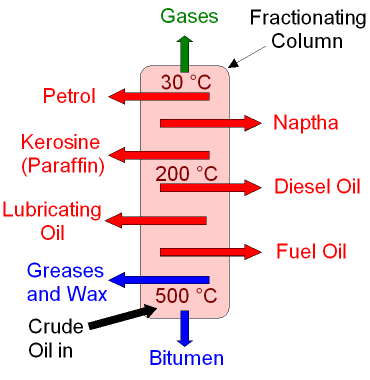
The fractional distillation of crude oil is shown in the picture below.
Crude
oil is heated until it
boils and then
the hydrocarbon
gases
are entered into the
bottom of the fractionating column.
As the gases go up the column the
temperature decreases.
The hydrocarbon gases condense back into
liquids
and the fractions are removed from the sides of the
column.
The different fractions
have different
uses.
The smaller the hydrocarbon molecule,
the further
it rises up the column
before condensing.
The fractionating column operates
continuously.
The temperatures shown are
approximate.
A sample of crude oil may be separated in the laboratory by
fractional distillation. The collection vessel is changed
as the temperature rises to collect the different
fractions.
![]() Links Hydrocarbons Revision Questions
Links Hydrocarbons Revision Questions ![]()
gcsescience.com The Periodic Table Index Hydrocarbons Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.