H2(g) + Cl2(g)
gcsescience.com 25 gcsescience.com
What are Bond Energy Calculations?
You can calculate the overall change
in energy for a
reaction
by using the values for the individual bond energies.
In the reaction between hydrogen and chlorine,
hydrogen
+ chlorine
![]() hydrogen
chloride.
hydrogen
chloride.
H2(g) + Cl2(g) ![]() 2HCl(g)
2HCl(g)
The reactants are hydrogen and chlorine.
The product
is hydrogen chloride.
You must put
energy in to break the bonds in
the reactants
before energy is given out by forming
new bonds in the products.
The minimum
amount of energy you need to put
in
to make the reaction happen is called the activation energy.
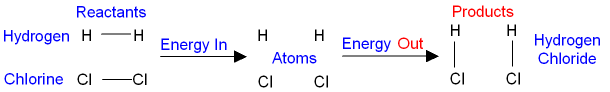
When the bonds between the hydrogen and
the
chlorine
molecules (the reactants) have been broken,
then both
hydrogen and chlorine exist as atoms.
When an atom of hydrogen and
chlorine react
with each other they make a new bond,
give energy
out, and form the product
hydrogen chloride.
What is the Bond Energy?
The bond energy is the
amount of energy which
1) you must put in to break the bond,
or
2) is given out when the new
bond is formed.
The table below shows some bond
energies which are
given in kilojoules
(thousand joules) per mole of
bonds.
| Bond | Bond Energy (in kJ per mole) |
| H—H | 436 |
| Cl—Cl | 242 |
| H—Cl | 431 |
In the reaction between hydrogen and chlorine, the big numbers
used to balance the equation
(1H2
+ 1Cl2 ![]() 2HCl)
2HCl)
tell you how many
moles there are of reactant
and product.
You must break
1 mole of H—H bonds and
1 mole of Cl—Cl bonds.
The amount of energy needed
(from the above table) is
436 + 242 kJ
= 678 kJ.
When 2
moles of H—Cl bonds are formed,
the amount of energy given out
is
2 x 431 kJ
= 862 kJ.
The
difference between the two amounts of energy
(reactants minus products) is the overall
amount of energy given
out per mole of reactants.
678 - 862 kJ = -184
kJ.
The change in
energy during a reaction is given
the symbol ΔH.
In the reaction between hydrogen and chlorine,
ΔH =
-184 kJ per mole.
For an exothermic reaction, ΔH is negative.
![]() Links
Catalysts and Energy
Enzymes
Revision Questions
Links
Catalysts and Energy
Enzymes
Revision Questions
![]()
gcsescience.com The Periodic Table Index Energy Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.