gcsescience.com 7 gcsescience.com
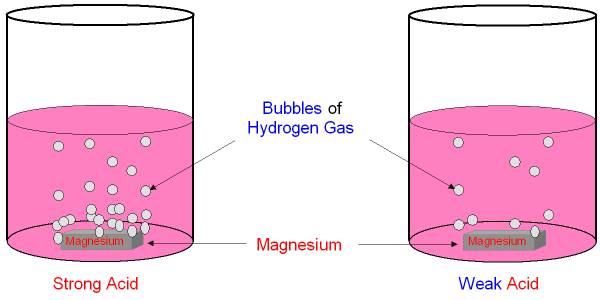
Strong and Weak Acids have Different Reaction Rates.
Magnesium will react
with an acid and make hydrogen gas.
The more concentrated the acid, the faster the magnesium will react
and you will see bubbles of hydrogen
being produced more quickly.
A strong
acid and a weak acid of the same
concentration
will react at
different rates with the same metal.
You can see the difference between a strong acid and a weak acid
of the
same concentration by looking at the reaction with
magnesium.
The strong acid reacts faster and you see
more bubbles of hydrogen.
This is because the strong acid has
more hydrogen
ions in the solution
even though it is at the
same concentration as the weak acid.
A piece of magnesium the same size
should be
used in both acids for the comparison to be fair.

The weak acid will
produce the same amount of
hydrogen as the strong acid
from the same amount of magnesium but the weak acid will take longer to do it.
![]() Links
Acids
Revision Questions
Links
Acids
Revision Questions
![]()
gcsescience.com The Periodic Table Index Acid Quiz gcsescience.com
Home GCSE Chemistry GCSE Physics
Copyright © 2015 gcsescience.com. All Rights Reserved.